PROCESSES AT THE ELECTRODES
Ion transport between electrolyte and electrode
Anode and cathode
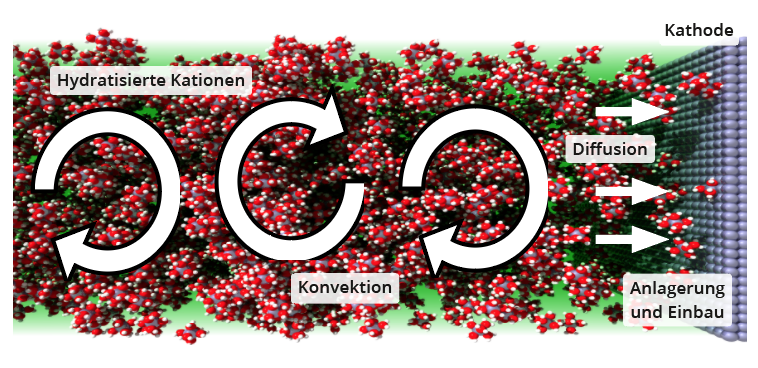
All the transport mechanisms between electrolyte and electrodes described below apply equally to transport away from the electrode and towards it. Therefore, the transport of cations to the cathode is described for both transport directions (see diagram below).
Convection in the volume
The diffusion movement due to the thermal movement of the particles and also the drift movement in the electric field of the electrodes is too small to maintain a galvanic deposition at the required rate if the area in the immediate vicinity of the electrode is already depleted of suitable ions. Therefore, convection of the electrolyte is essential. Such macroscopic circulation of all particles in the electrolyte does not necessarily have to be imposed from outside , but already takes place due to spatial temperature and thus density differences in the electrolyte. However, convection alone cannot bring particles directly to the electrode, as a diffusion boundary layer always forms in moving liquids above solids, the strength of which decreases with increasing convection speed.
Diffusion in the boundary layer
The particles directly at the electrode surface adhere to this, i.e. they are physically bound to it relatively weakly by various interaction forces. Above this, layers of particles stacked parallel to the electrode surface can be imagined, each of which can move parallel to the previous layer with a certain difference in speed. From a certain distance, this diffusion layer merges into the convective region of the electrolyte . Within the diffusion layer, the concentration of ions, which are consumed at the electrode through growth (e.g. Cu+ + e- → Cu) or phase transformation (e.g. 2 H+ + 2 e- → H2) , decreases in the direction of the electrode, which acts as a "particle sink". This concentration gradient as a driving force is all greater the thinner the diffusion layer, i.e. the stronger the convection velocity above it. Strong convection therefore also promotes diffusion through the diffusion boundary layer.
Attachment to the electrode
Cations are always hydrated in aqueous solutions, i.e. surrounded by a hydration shell of water molecules aligned to the ion due to the ion-dipole interaction. This shell must first be stripped off before the cation can attach to the cathode.
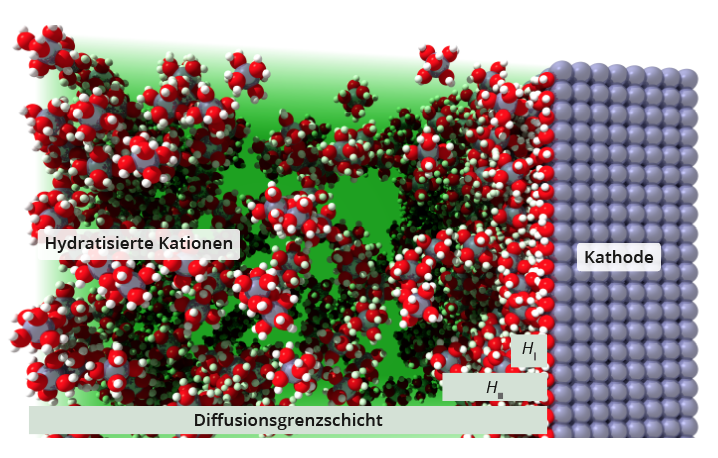
A closer look at the diffusion boundary layer
Two areas can be defined within the diffusion boundary layer :The inner Helmholtz layer refers to the monolayer of solvent molecules (e.g. H2O) or other ions of the electrolyte adsorbed on the electrode .The outer Helmholtz layer consists of the hydrated ions of the electrolyte, which are attached to the inner Helmholtz layer. In order to pass from the electrolyte to the surface of the electrode (or to pass from the electrode into solution ), the ions must penetrate the Helmholtz layer, which is referred to as the passage reaction. The ions then strip off their hydration shell and are incorporated into the lattice of the solid. The passage through the boundary layer as well as the stripping of the hydration shell requires an activation energy, which must be applied to the external voltage source by an increased voltage (passage overvoltage).
Layer growth
Physical principles
After the cations have reached the cathode and stripped off their hydrate shell , they initially attach themselves loosely bound as Ad atoms to the surface of the solid. They can diffuse there thermally activated until they are permanently incorporated into the crystal structure at an energetically favourable location .
Simulation...
The following simulations represent the mechanism of addition and incorporation of a cation into the solid in a very simplified way: Each particle in a cubic lattice has 26 neighbour sites (6 across the side faces, 12 across the edges, and 8 across the corners of the central cell), each of which can be occupied or unoccupied. The more occupied neighbours a particle has (whereby the neighbouring sites via the edges and corners contribute less due to the greater distance), the greater its binding energy at this location. The probability p for a thermally activated change from a lattice site (energy E1) to a neighbouring site (E2) is therefore:
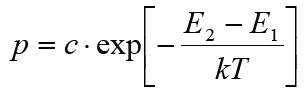
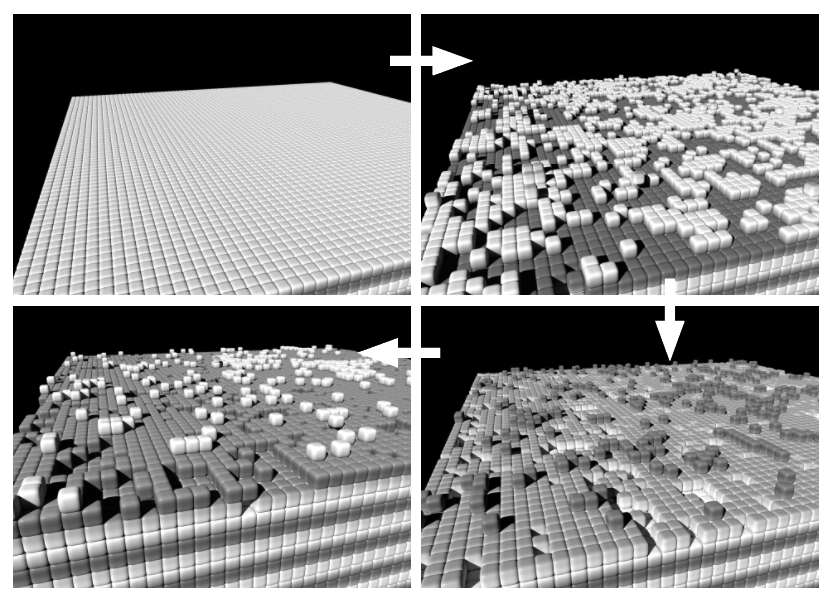
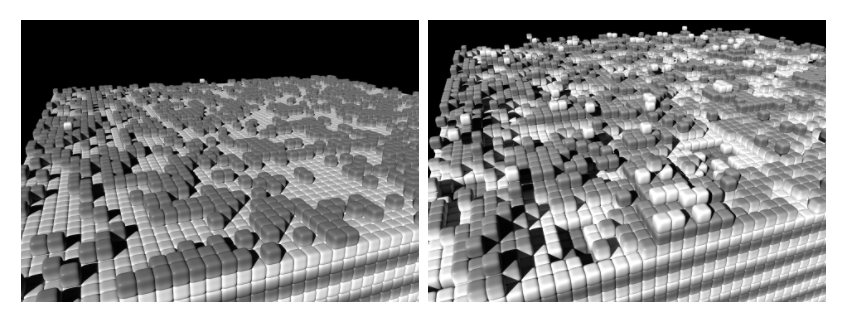
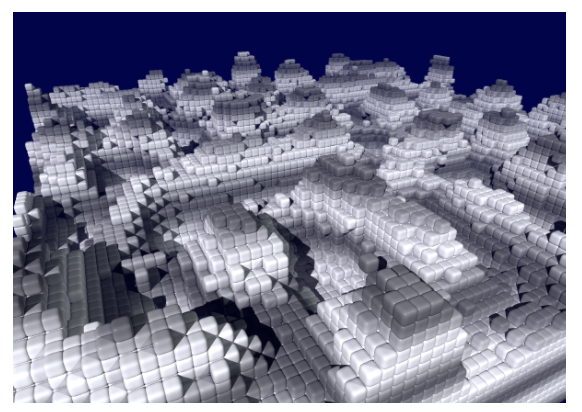
...and practice
The reality of electrodeposition is much more complex than the assumptions of the simulations discussed here:
electrodes
are generally not single crystals, but amorphous or
nanocrystalline solids, which means that the bonding conditions on their
surface vary locally.
transport mechanisms within the diffusion boundary layer, in particular the
passage through the inner and outer Helmholtz layer, have also not been taken into account. The
stripping of the hydrate shell and chemical reactions also have an effect on the
energy balance of a particle between deposition and incorporation into the
crystal structure.
Further Information:
> Application areas and compatibilities
> Image Reversal Resist Processing
Filter products


















