ELECTROCHEMICAL FUNDAMENTALS
The metal potential
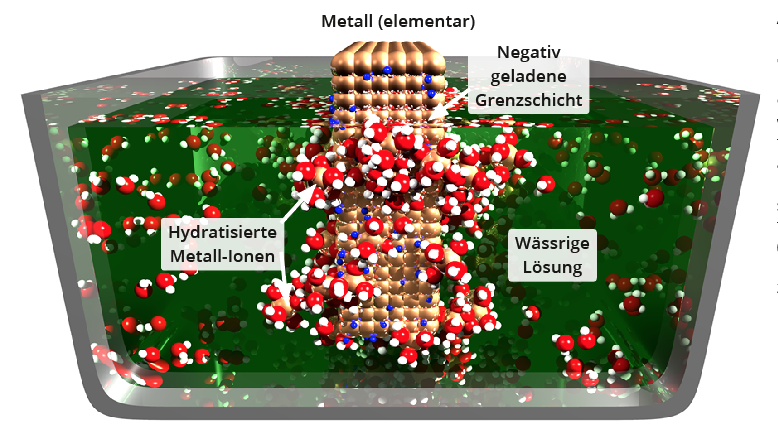
A metal in an aqueous solution
If a metal is immersed in an aqueous solution (a salt solution, diluted acid or just water), part of the metal goes into solution as positive ions, whereby the metal becomes negatively charged (diagram below).
The system of metal and solution strives for minimum free enthalpy H=U - T-S (U=internal energy, T=temperature, S= entropy). The change in internal energy concerns i) the lattice energy of the metal atom when it leaves the solid and ii) the energy released during hydration of the dissolved ion .
The higher degree of freedom (=increase in entropy) of a metal ion previously bound in the solid in solution, and the greater localisation (=decrease in entropy) of the water molecules bound to the metal ion during hydration contribute to the change in entropy.In equilibrium, the two phase transitions, the dissolution of the metal atoms and their reintegration into the solid, are in balance. The potential difference between the dissolved phase and the solid in the equilibrium state is called the metal potential.
The electrochemical voltage series
Theory
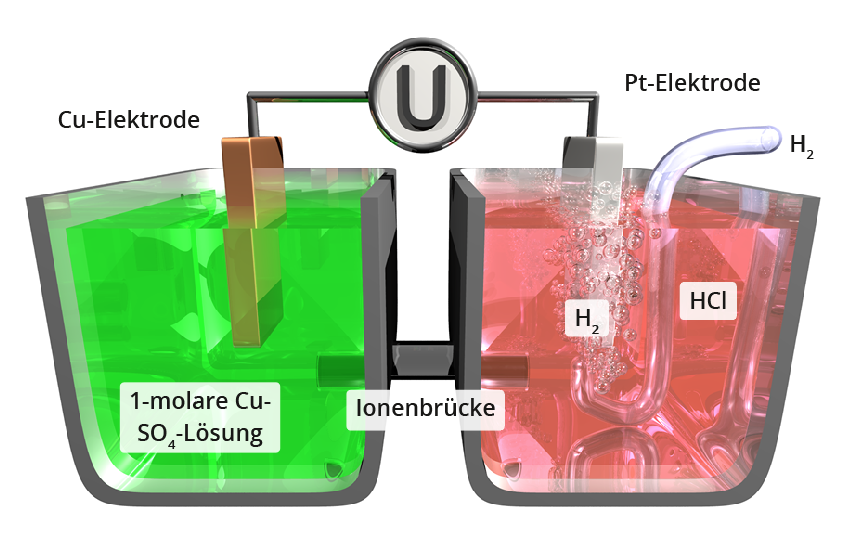
The metal potential between the negatively charged electrode and the electrolyte enriched with positive metal ions cannot be measured even , whereas the potential between two different electrode potentials can. In order to compare the relative size of the metal potentials of different metals, a measurement is carried out with a counter electrode under standard conditions (25 °C, 101.3 kPa, one-molar solution of the analysed metal).
The counter electrode is a platinum electrode surrounded by H2, on whose catalytically active surface the H2 gas dissociates to form atomic hydrogen, which forms an atomic layer on the Pt electrode (normal hydrogen electrode).
Standard potentials and their meaning
If such a measurement is carried out under standard conditions for certain elements (see diagram above), their standard potentials are obtained, which are listed in tabular form on the following page . The higher the standard potential of a metal, the more precious the metal is: metals with a positive standard potential are by definition precious metals that are not attacked by acids (exception: oxidising acids). Base metals with negative standard potential dissolve in acids to form H2.
Charge exchange
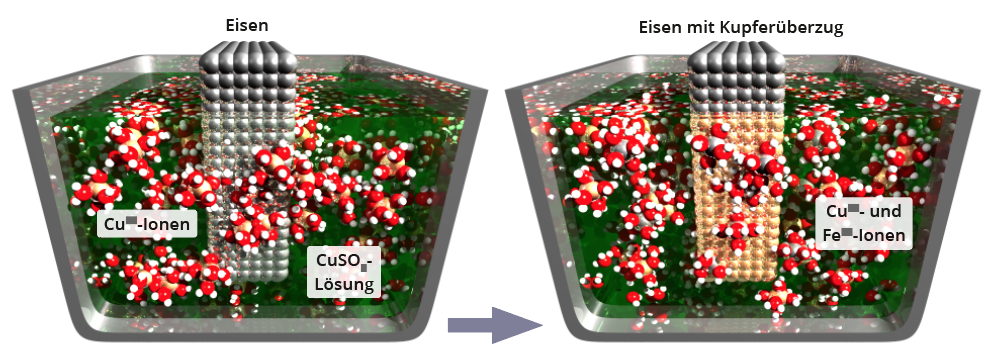
Immersion deposition: A metal in the salt solution of a more noble metal
If a less noble metal such as iron is immersed in an aqueous solution of a metal salt (e.g. copper as a CuSO4 solution) (see diagram below), atoms from the iron first pass into solution as ions (as described in the section The metal potential). For each dissolved Fe2+ ion, two electrons remain in the iron, which combine with a Cu2+ ion from the solution to form elemental copper. This copper deposits as a thin film on the iron, preventing the further transfer of iron ions into solution and ultimately stopping the process of charge exchange between iron and copper.This electroless mechanism of charge exchange is used on a large scale, for example in the copper plating of iron, or in the silver plating of copper or brass in a silver nitrate solution.
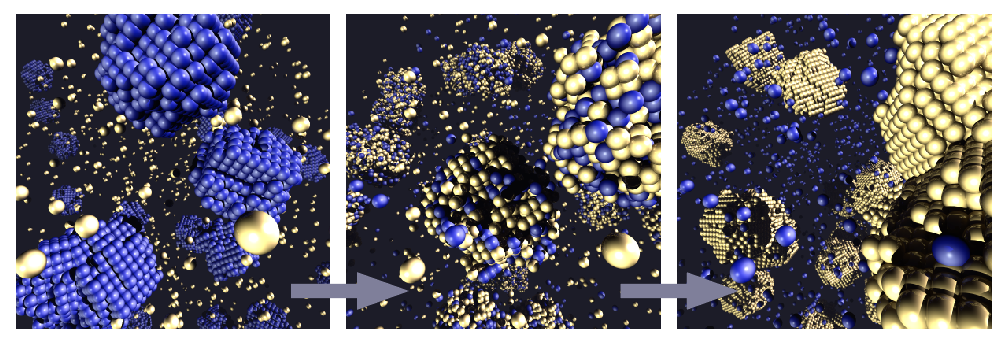
Cementation: A metal powder in the salt solution of a more noble metal
If the principle of immersion deposition is not applied to a metal rod, but to a powder of a metal in a salt solution of a more noble metal, the transformation may be complete: If the size of the metal particles of the powder is comparable to the thickness of the dissolved layer or the thickness of the coating of the more noble metal, the less noble metal particles dissolve completely, and serve until then as a substrate for the growth of a particle of the more noble metal.
In this way, for example, elemental copper can be obtained with iron from a copper solution, or elemental gold from a cyanide gold solution with zinc dust (diagram below).
The galvanic element
Two metals in an acid
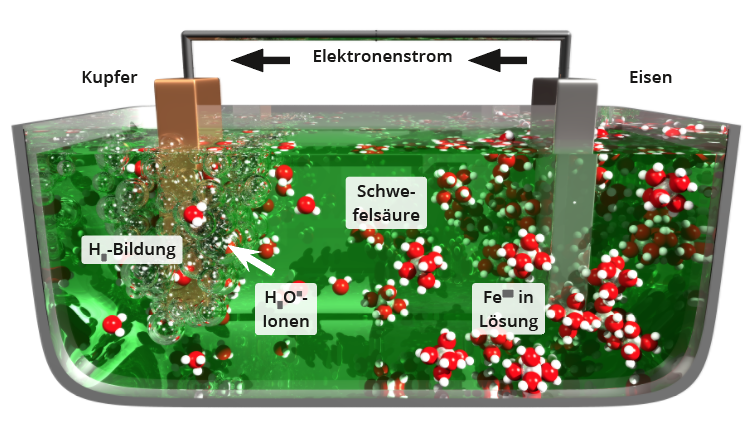
If, as shown in the diagram below, two different metals (one less noble than the other, in this example iron and copper) are immersed in an acid (in this example sulphuric acid) in an electrically conductive connection, this is referred to as a galvanic element. From the time of immersion in the acid, the following electrochemical reactions take place:Iron ions pass from the electrode with the less noble metal, in this example iron, via Fe -> Fe2+ + 2e- into solution. The two electrons migrate via the electrical connection between the two electrodes to the more noble copper electrode, where they react via 2 H3O+ + 2e- -> 2 H2O+ H2 oxonium ions of the acid to form neutral hydrogen, which rises in gaseous form. The reaction only comes to a standstill when either the iron electrode or the acid is exhausted. As long as the reaction takes place, the measurable voltage between the two electrodes is the difference between the standard potentials of the two metals (in this example approx. 0.8 V), which is the basic principle of a battery.An undesirable effect of the principle of galvanic elements is electrochemical corrosion: If components made of e.g. iron and aluminium, or iron and copper are installed together in an electrically conductive manner, the less noble metal begins to corrode as soon as the components are wetted by (e.g. rain) water.
Further Information:
> Application areas and compatibilities
> Image Reversal Resist Processing
Filter products


















